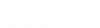California Initiative to Advance Precision Medicine Important Information to know about Clinical Research
Any person considering volunteering in a clinical trial and is asked to give his, her, or their consent should be informed of the many important participant rights and responsibilities
What is Informed Consent?
All participants are entitled to understand all aspects of a study’s protocol. Everyone has the right to decide to participate in clinical research.
Informed consent is the ethical responsibility of the study researcher to guide and facilitate a patient’s understanding of a clinical trial’s protocol and define the roles and expectations of you as a voluntary study participant.
What is the goal of informed consent?
Informed consent makes sure that individuals understand all aspects of a study, feeling empowered to decide whether they are comfortable with the study risks and benefits. Individuals can then decide if they want to participate in a study.